Medical News
Cancer-Killing Virus Injected to the First Human Patient in New Clinical Trial
A recent clinical trial has started evaluating the safety and tolerability of a novel therapy involving a virus that can infect and kill human cancer cells.
Overview
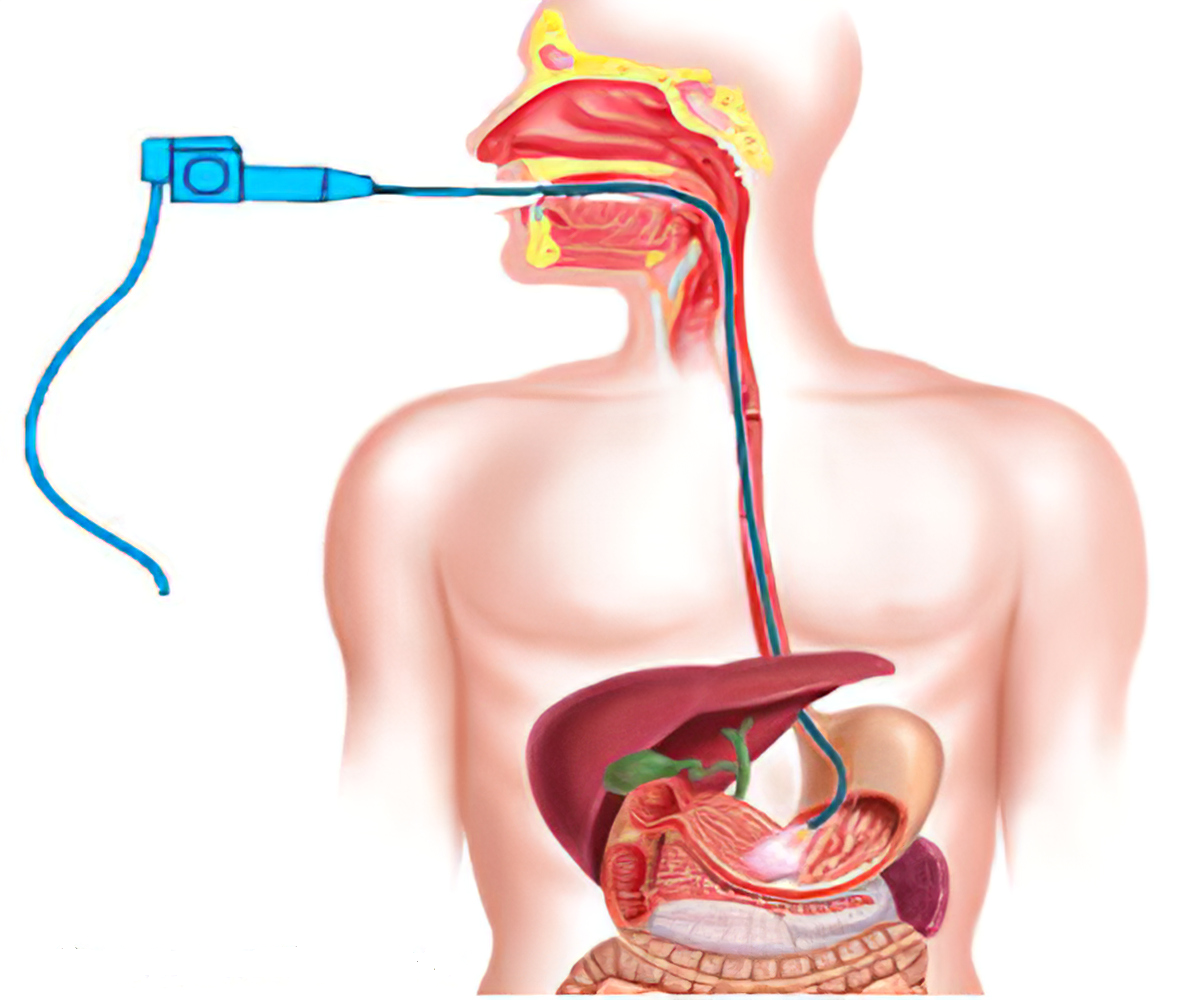
A recent Phase 1 clinical trial has administered a dose of an experimental anticancer drug called CF33-hNIS, or Vaxinia, to the study’s first participant. This novel therapy involves using an oncolytic virus, a type of virus that can infect and kill cancer cells without harming healthy tissue.
Vaxinia, a genetically modified smallpox virus, has been previously shown to be effective against a broad range of cancers in laboratory and animal models. This clinical trial conducted by City of Hope, a cancer research and treatment institute in the United States, in collaboration with Imugene, a biotech company in Australia, will test the novel oncolytic virus in cancer patients with advanced solid tumors. (Source)
Laboratory studies suggest that Vaxinia may be more effective than the previous generation of oncolytic viruses in reducing the size of tumors, making this therapy especially promising. (Source)
The Department of Surgery at City of Hope said “The particular importance of CF33/ Vaxinia is that this virus is designed to target all types of cancers. It is one of the first of a new generation of therapeutic viruses that would be much more potent than prior viruses, and it is potentially more selective for cancer while able to spare normal tissues.”
The CEO of Imugene said “We are keen to revolutionize cancer therapy, and no longer are we satisfied with incremental improvements in survival, we want to cure patients. By making cancer into one disease and having a targeted agent to obliterate it, that’s the holy grail of cancer therapeutics!”
Oncolytic viruses
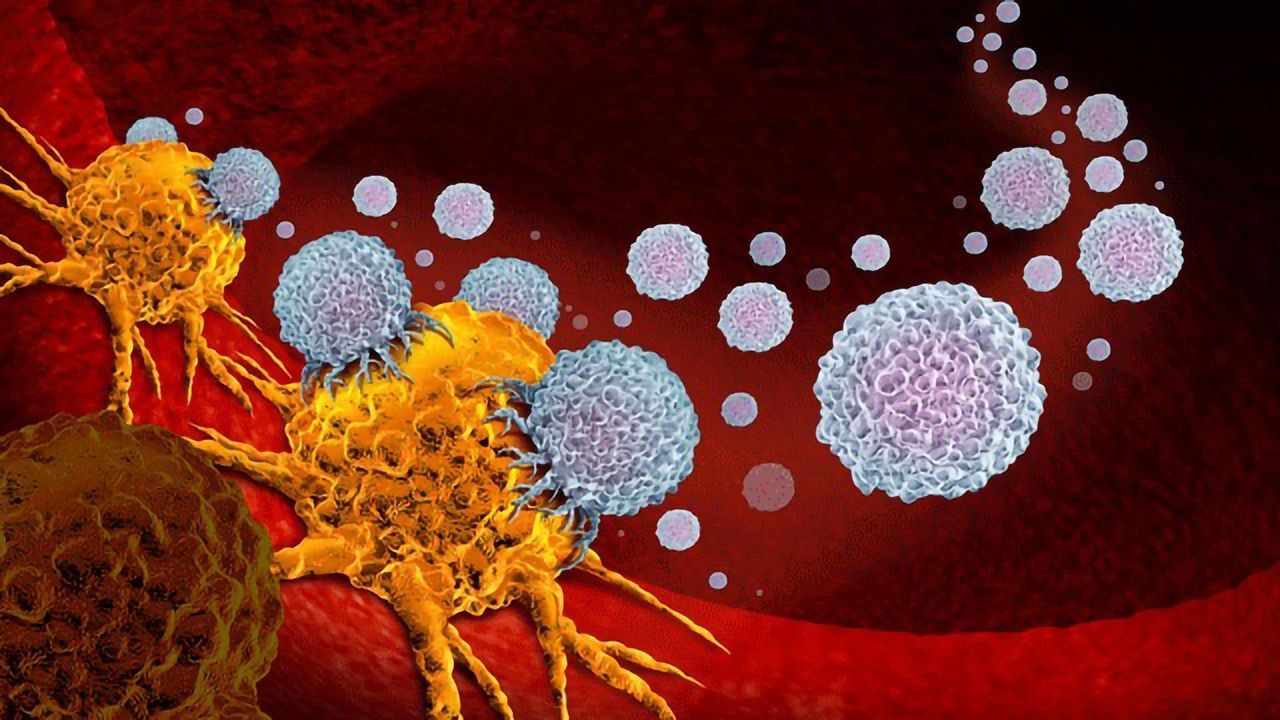
Oncolytic viruses include viruses found in nature or are genetically engineered to selectively infect and replicate in tumour cells.
As oncolytic viruses replicate, they can disintegrate and kill infected tumour cells. When tumour cells burst, they release tumour proteins or antigens, which the immune system recognizes as foreign. The immune response then elicits against these antigens resulting in further death of tumour cells.
Additionally, the immune system’s ability to recognize the tumour cells creates a memory against the tumour antigens, which can help prevent cancer recurrence. Besides providing durable protection, a small dose of oncolytic viruses can be effective against the tumour due to the ability of the virus to replicate and spread in the tumour cells.
Cancer cells express proteins and receptors on their surface distinct from healthy cells that help them evade the immune system, metastasize, and prevent cell death. Oncolytic viruses use these cancer cell-specific proteins and receptors to target them.
Interestingly, the same characteristics that eventually make cancer cells resistant to chemotherapy or radiation treatment actually enhance the success of oncolytic viruses, such as CF33-hNIS.
Moreover, the proteins targeted by oncolytic viruses are often common to a broad range of cancers, making these viruses a versatile tool.
Using Vaxinia to target tumour cells
CF33-hNIS or Vaxinia, developed by the researchers at City of Hope is a genetically modified version of the vaccinia or smallpox virus. The researchers have designed CF33-hNIS to enhance its ability to replicate in tumour cells, facilitating a large immune response against the tumor cells.
In addition, the modified vaccinia virus also expresses a protein called human sodium iodide symporter (hNIS), which transports iodide ions into the cells. Thus, tumour cells infected by the virus express hNIS, allowing radioactive iodine uptake.
Imaging techniques such as Positron Emission Technology (PET) scans can then be used along with radiolabeled iodine as a dye to help track the distribution of the virus in the body and its effectiveness.
Moreover, hNIS can also help selectively target tumour cells that accumulate radioactive iodine using radiotherapy.
Clinical trial design
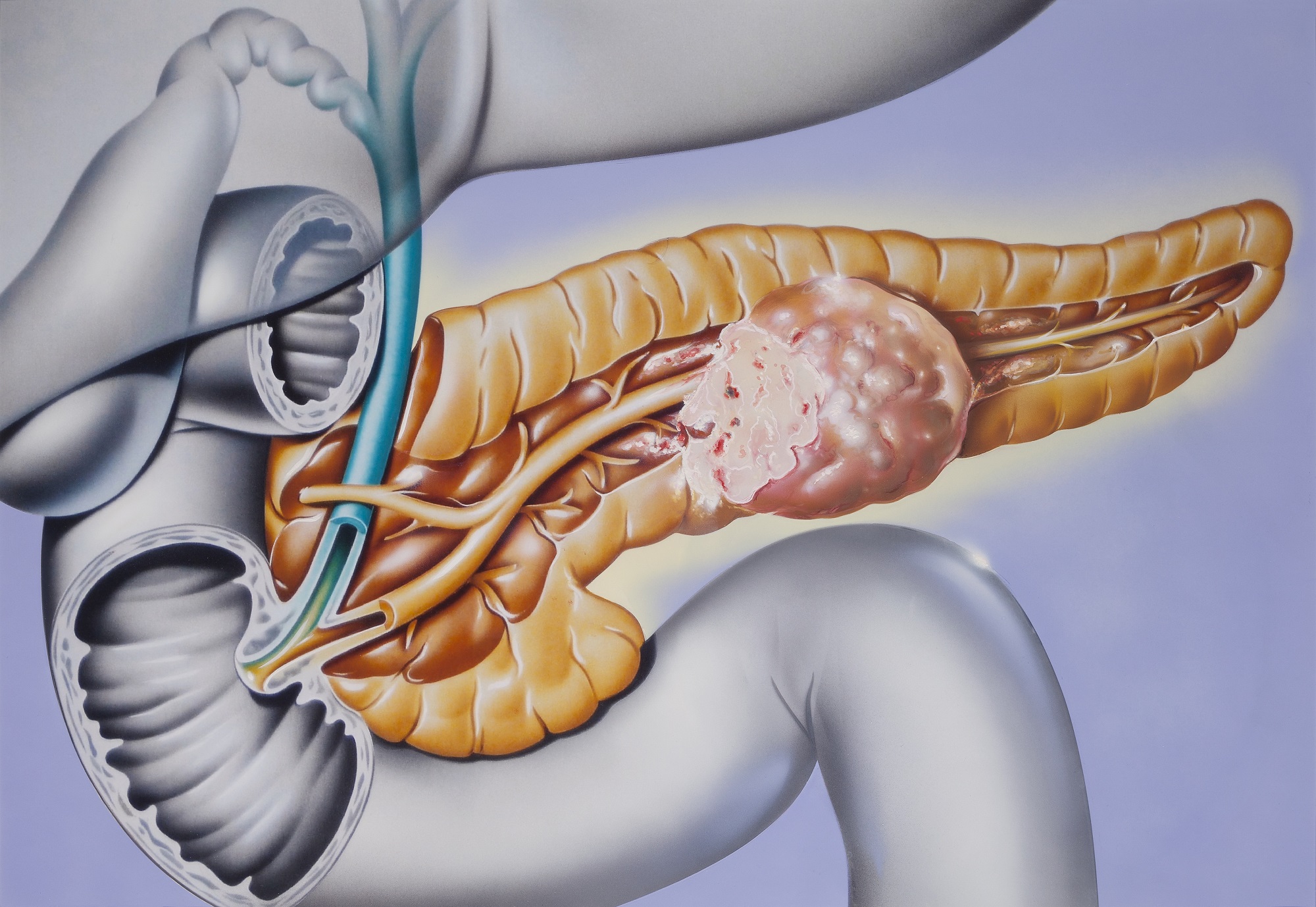
Previous studies have shown that CF33-hNIS is effective against cell culture and animal models of breast, colorectal, pancreatic, ovarian, and lung cancers. During the Phase 1 clinical trial, researchers will test the safety and tolerability of CF33-hNIS in cancer patients by injecting the virus directly into the blood or the tumour.
Specifically, the trial will include about 100 cancer patients with metastatic or advanced solid tumours who have previously received at least two standard cancer treatments.
Upon successful demonstration of Vaxinia’s safety, the researchers also intend to test treating tumour cells using a combination of this oncolytic virus with many other immunotherapies. Researchers are hoping the CF33/ Vaxinia platform will move rapidly to clinical testing in combinations with these and become effective combination immunotherapies in the treatment of human cancer.
The researchers also intend to examine the efficacy of this therapy as a secondary outcome throughout this phase 1 trial.
Reference
- https://clinicaltrials.gov/ct2/show/NCT05346484
- https://link.springer.com/article/10.1186/s12967-018-1483-x
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5600978/
- https://aacrjournals.org/mct/article/20/1/173/92921/Recombinant-Orthopoxvirus-Primes-Colon-Cancer-for
- https://www.eurekalert.org/news-releases/953071